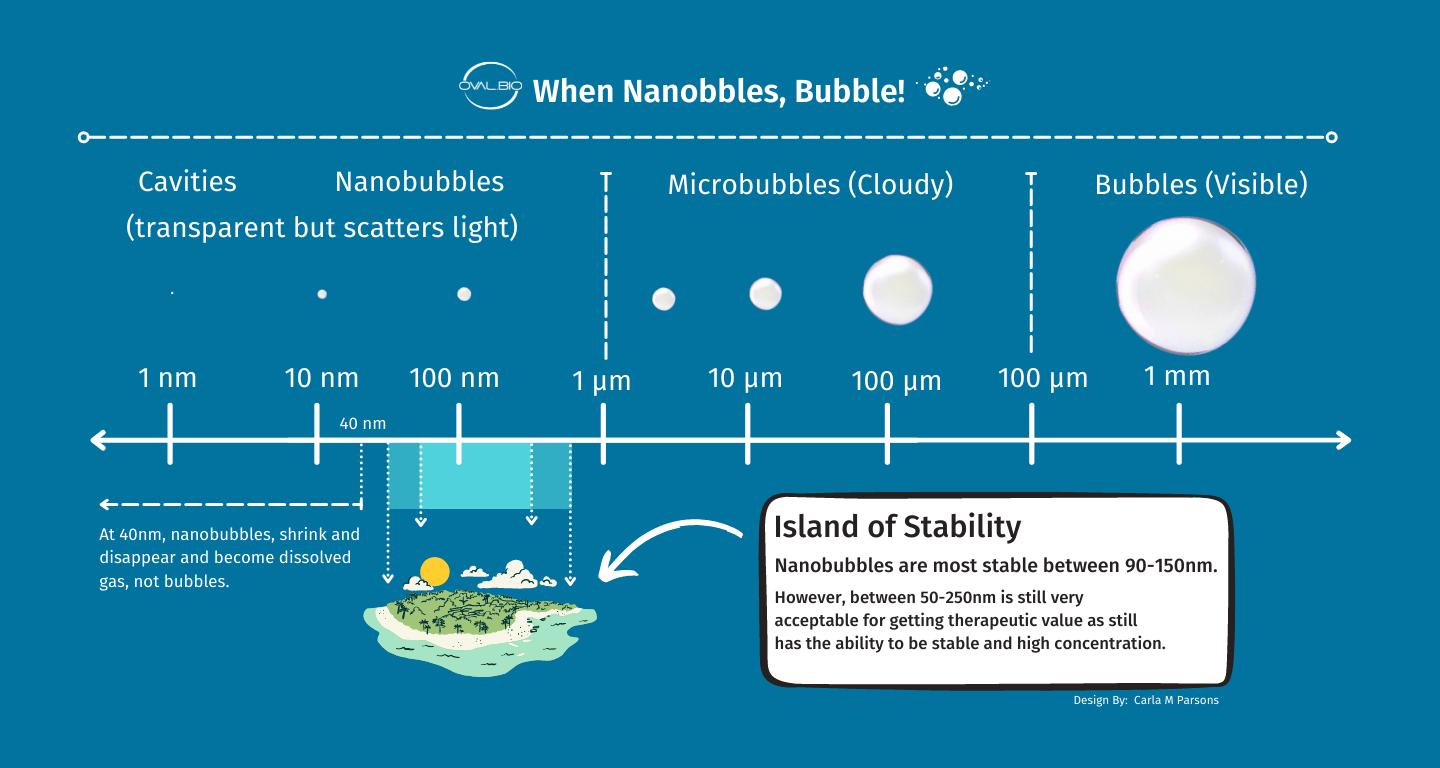
Nanobubbles are very small bubbles of gas in a liquid. These bubbles are in the nanoscopic range in that they are 1000 nm or less in diameter. For the purpose of nanobubble therapy however, the ideal size (“island of stability”) is 50 nm – 200 nm in diameter, with the average size of each bubble at about 100 nm, 500 times smaller than a microbubble. For some perspective, an average human hair is 100,000 nm– 1,000 times wider than a single 100 nm bubble.
GRAPHIC 1
GRAPHIC 2
Nanobubbles are considered an effective therapeutic because of their unique physical and chemical properties.
Physically: Nanobubbles possess the ability to stay in water for long periods of time (months or even years) whereas microbubbles or larger bubbles only stay in water for hours (think how fast your soda loses its carbonation). Staying in water for such long periods of time is advantageous when creating packaged goods such as oxygenated nanobubble water like O2n water.[1] In addition, a valuable technical advantage that is gained by this stability is the ability to cycle the same water through the nanobubbler multiple times, thereby increasing the nanobubble concentration, which is crucial to get therapeutic value. There are several reasons for nanobubble’s ability to stay in water:
Size – Nanobubbles’ nanoscopic size allows for their buoyancy to be almost exactly equal to gravitational forces, this creates the equal up and down forces which lets nanobubbles stay suspended in liquid for months or years. This is in contrast to larger bubbles, whose large buoyant forces overcome gravity and push them upward, forcing them to leave the water (think of blowing bubbles in water with a straw). What this means is that any sort of coalescence (combining) of bubbles is detrimental to the lifespan and efficacy of nanobubbles.

GRAPHIC 3
Due to their size, nanobubbles undergo Brownian motion, a random movement in and around water, evenly distributing them throughout the water.
GRAPHIC 4
Nanobubbles’ strong negative charge causes bubble-to-bubble repulsion, preventing coalescence. This repulsion is linked to zeta potential, highly correlated with nanobubble stability, lifespan, and potential for increased concentrations.
To delve into zeta potential or the optimization of various nanobubble properties for maximum therapeutic benefit, refer to [2].
Nanobubbles’ extremely large internal pressure prevents bubbles from losing surface charge, enabling them to maintain zeta potential and a long lifespan ([1]).
Chemically: Liquids like water have a limited capacity for dissolved gas, known as the saturation point. Nanobubbles, acting as small oxygen cavities separate from water, don’t contribute to water’s saturation point. Instead, they can supersaturate water, increasing partial pressure by more than five times, as shown in peer-reviewed articles ([2]).
Graphic 5
The following graph shows water saturated with nanobubbles vs microbubbles vs control. The partial pressure is highest (by a significant margin) for fine microbubbles/nanobubbles.[2]
Graphic 6
I generated fine micro/nanobubbles using a dedicated micro/nanobubble aerator, with an oxygen gas supply of 1.5 L/minute for 15 minutes, followed by brief sonication. Additionally, I produced oxygen macrobubbles in 150 mL of ultrapure water using porous ceramic with an oxygen gas supply of 1.5 L/minute for 15 minutes. The oxygen partial pressure in ultrapure water underwent measurement through blood gas analysis. The data represent the mean ± standard error of the mean from five separate experiments, each performed in duplicate. Significance level: **P<0.01.” [2]
In contrast, microbubbles, for instance, ascend to the water’s surface, offering limited therapeutic value. In simpler terms, nanobubbles enhance water’s oxygen concentration by allowing it to reach its maximum dissolved oxygen content, combined with the oxygen within the nanobubbles. In comparison, microbubbles escape the water too quickly and, therefore, cannot contribute to transdermal oxygenation.
We detail this concept even more in our optimization article.[3]
Nanobubble immersion therapy’s simplicity lies in immersing a user in nanobubble-saturated water. However, its therapeutic value hinges on precise execution. At oval.bio, our emphasis centers on optimizing two crucial factors for nanobubble efficacy: bubble size and concentration.
To ensure precision in size, it’s critical for bubbles to align within the island of stability, ranging from 50 nm to 200 nm. Bubbles smaller than 50 nm dissolve into oxygen, escaping if water is maximally saturated. Conversely, bubbles larger than 200 nm coalesce and swiftly exit the water.
Refer to the figures below for the diameter range our nanobubbler achieves (mean 115.9 nm and mode 87.4 nm).
GRAPHIC 7
Smaller nanobubbles enable superior water saturation with a higher oxygen volume, akin to the efficiency of packing sand into a bottle compared to pebbles. This density advantage, similar to the nanobubbles, offers practical benefits.
Practically, it’s advantageous to target the lower end of the stability island, precisely what our nanobubbler achieves.
Concentration Dynamics: Elevated oxygen concentrations in water compared to the body facilitate oxygen absorption through osmosis. This transfer is more effective with higher oxygen levels in water, leading to increased oxygen quantities absorbed by the body. Nanobubbles elevate water’s dissolved oxygen potential, enhancing the saturation of the body with a higher oxygen quantity.
Sources:
[1] https://pubmed.ncbi.nlm.nih.gov/22985594/
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4181745/
[3] https://www.sciencedirect.com/topics/neuroscience/osmosis